Bacteria battle: How one changes appearance, moves away to resist the other
Two types of bacteria found in the soil have enabled scientists at Texas A&M AgriLife Research to get the dirt on how resistance to antibiotics develops along with a separate survival strategy. The study, published in the journal PLOS Genetics this month, identifies an atypical antibiotic molecule and the way in which the resistance to that molecule arises, including the identity of the genes that are responsible, according to Dr. Paul Straight, AgriLife Research biochemist.
Straight and his doctoral student Reed Stubbendieck observed a species of bacteria changing its appearance and moving away from a drug to avoid being killed.
Straight's lab on the Texas A&M University campus in College Station in general focuses on understanding how communities of bacteria interact with each other and other microbes.
"Over the past few decades, scientists have come to understand that bacteria aren't just single individual cells that somehow cause infections or degrade toxins, for example," Straight said. "In fact, they are populations and communities of many, many cells, whether just a single species of bacteria or a very diverse community. We are most recently aware of this in terms of the human microbiome. People have more bacteria cells in them than they have human cells."
But what has not been fully understood about bacteria and microbes in general, he said, is the way in which they form these types of communities with more than one species.
"It's both an ecological and a mechanistic bacteriology question," Straight said. "For nearly 100 years, we've known that bacteria can produce molecules that can block the growth of other organisms including other bacteria, and those molecules have been very useful as antibiotics."
Straight said the common understanding of the usefulness of antibiotics, however, sidestepped the ecological dynamics of the bacteria themselves in how they form communities, and interact with each other.
"We wanted to know what happens when we put two bacterial species together to compete with each other and use that model as a way to identify new molecules, identify pathways, or gene functions, that are required for the bacteria to survive under competitive stress," he explained. "Identification of interesting new molecules or bacterial mechanisms of control that one might exploit can lead to developing a new antibiotic."
For this study, Stubbendieck put together two species of non-pathogenic, soil-borne bacteria, Streptomyces and Bacillus subtilis, in different ways in the laboratory. He monitored the bacteria for different patterns in growth, motility and other factors when the organisms were together as opposed to when they were separate.
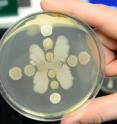
Stubbendieck noticed that the two bacteria would grow as expected in each colony initially, but over time one of the bacteria colonies would start to destroy the other one.
"It was very visual," Straight said. "It would cause lysis, meaning that the cells inside the dying colony would be dissolved, leaving a mark of where this had happened."
Stubbendieck had to identify the molecule or other functions that are responsible for causing the destruction, thus the way in which resistance might emerge.
"The molecule turned out to be very strange. It doesn't look like any of the familiar antibiotics," Straight said. "We find it interesting, because its chemical structure suggests it's probably functioning in a way that is very different from the common antibiotics that are used."
Stubbendieck also noted that in the region where the cells were destroyed, there developed "little teeny colonies of bacteria" growing, indicating that they're resistant to the molecule. So he picked a number of those colonies and sequenced the genomes, which found the mutations that cause resistance.
"I put a bunch of the cells with mutant bacterial stains on a petri dish together, and when I came in the next morning and looked in the incubator, I saw a difference between the mutants and the non-mutant strain that was night and day, and we knew we are on to something," Stubbendieck recalled.
"With two pieces of the puzzle -- the molecule itself identified plus a way in which the resistance to that molecule would arise, including the identity of the genes that are responsible for resistance -- Reed was able to dissect the pathway of resistance," Straight said. "And it turns out that in a B. subtilis membrane, proteins work as signaling systems for lots of different things. They can receive signals from the external environment, signals from other bacteria, signals telling them about the status of their cell in a fluctuating environment.
"If something damages a membrane, bacteria have a way of sensing that and then turning on the response," Straight said.
All of the mutations Stubbendieck identified were in the same gene that encodes for a protein in the membrane that functions like a signaling protein, or it has a partner that it talks to, and all mutations turned on the signaling system. And, because the mutants had proteins that were turned on all the time, the drug that previously would have been effective could no longer kill the bacteria.
Additionally, not only did the researchers see that resistance could emerge that way but also the population of the B. subtilis, the one that's typically killed by the drug, changed in appearance.
"It had morphological shapes and structures to it, which suggested that this organism had undergone a really profound change. That allowed it not only to be resistant to this drug, which causes lysis, but also to move as a population of bacteria across the agar surface in a petri dish," Straight said.
"This shows a way that organisms can interact with each other in a competitive, dynamic environment that's very different from the way we typically think about antibiotics," he added. "It is not just a simple, one-way street of a molecule that's produced and causes growth inhibition of the pathogen, and the pathogen can become resistant and that might be a problem for health reasons. What we're seeing here are molecules that can function like an antibiotic and cause something like lysis, or cell death. And the organisms can use not just one resistance function but a combination of responses as a way of circumventing a competitive crisis."
"This helps scientists build a much more mechanistically detailed picture of the competitive dynamics between bacteria, which helps us understand what happens in soil or inside a human intestine," Straight added. "It helps us start to get a better image to work from when we talk about the role of microbes in the environment and the way competitive interactions structure microbial communities; how something becomes resistant and therefore how we might control that."
Source: Texas A&M AgriLife Communications
Other sources
- Spread of antibiotic-resistance gene does not spell bacterial apocalypse — yetfrom News @ NatureMon, 21 Dec 2015, 17:38:10 UTC
- Bacteria battle: How one changes appearance, moves away to resist the otherfrom Science DailyMon, 21 Dec 2015, 0:51:25 UTC
- Bacteria battle: How one changes appearance, moves away to resist the otherfrom PhysorgFri, 18 Dec 2015, 20:01:53 UTC
- Bacteria Battle: How One Changes Appearance, Moves Away to Resist the Otherfrom Newswise - ScinewsFri, 18 Dec 2015, 18:33:03 UTC