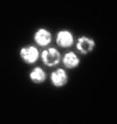
Under the microscope, strong-swimming swamp bacteria spontaneously organize into crystals
Related images
(click to enlarge)
Insects form swarms, fish school, birds flock together. Likewise, one species of bacteria forms dynamic, living crystals, says new research from Rockefeller University. Biophysicists have revealed that fast-swimming, sulfur-eating microbes known as Thiovulum majus can organize themselves into a two-dimensional lattice composed of rotating cells, the first known example of bacteria spontaneously forming such a pattern. "The regular, repeated arrangement of the microbial cells shares the geometry of atoms within a mineral crystal, but the dynamics are fundamentally different; the bacterial crystals constantly move and reorganize as a result of the power generated by individual cells within them," says study author Albert Libchaber, Detlev W. Bronk Professor and head of the Laboratory of Experimental Condensed Matter Physics.
The single cells' rotating motion -- which forms the crystals by drawing in other cells and then powers the crystals' own motion -- led the researchers to dub them "microscopic tornadoes" in a paper awaiting publication in Physical Review Letters.
It's no coincidence that Thiovulum majus is among the fastest swimming bacteria known. Capable of moving up to 60 body lengths per second while rotating rapidly, these microbes propel themselves using whip-like flagella that cover their surfaces. But in its natural habitat, deep in marsh water, these microbes don't travel much. They tether themselves to a surface and use their flagella to generate a current strong enough to pull in the nutrients they need: sulfides from rotting organic matter and oxygen used to burn the sulfides.
What one cell can do, many can do much better, and in previous work, Libchaber and postdoc Alexander Petroff examined how groups of tethered Thiovulum organize and reorganize themselves so as to pull in more nutrients.
"Because this microbe can generate so much force with its flagella, we became curious about what dynamics might emerge when many swim freely together," Petroff says of the investigation, which began when study co-author Xiao-Lun Wu, of the University of Pittsburg, was visiting. "After we put an enriched culture of Thiovulum under a microscope, this beautiful structure appeared."
Researchers set about determining the balance of physical forces that explain how the microbes organize themselves into crystals. When placed in an observation chamber within a microscope slide, the microbes swam either up or down until they collided with the glass. But even then, they kept on swimming, like flies trying to escape through a closed window, Petroff says.
Swimming in place, the cells draw water toward, then up and around themselves, creating a tornado-like flow. This flow pulls in nearby cells, which cluster together in a shifting two-dimensional lattice that is similar to the three-dimensional pattern that defines crystals. Within the lattice, each cell has six immediate neighbors, creating a hexagonal pattern that appears frequently in nature, including among penguins packing together for warmth and in the chambers of honeycomb. It is the densest way to pack things of a uniform size. But the bacterial crystal continues to reorganize and melt, animated by the cells' rotating motion, which causes the microbes to shift against one another, in much the same way that gravity pulls sand grains down the sides of a pile.
It's not clear why the microbes form these crystals, or even if they do so in habitats outside of microscope slides. But the coherent group behavior responsible for generating the crystals is a common phenomenon, known as collective dynamics.
"Usually, when birds, insects, fish, or even bacteria move together in a coordinated fashion, you see coherent motion on the scale of the group, but disorder at the level of the individual," Libchaber says. "This is not so for Thiovulum. Instead of turbulent movement, the individual cells form extremely regular crystalline structures. It appears that Thiovulum crystals represent a new form of collective dynamics."
Source: Rockefeller University
Other sources
- Under the microscope, strong-swimming swamp bacteria spontaneously organize into crystalsfrom Science DailyTue, 7 Apr 2015, 2:50:07 UTC
- Under the Microscope, Strong-Swimming Swamp Bacteria Spontaneously Organize Into Crystalsfrom Newswise - ScinewsMon, 6 Apr 2015, 22:00:16 UTC
- Under the microscope, strong-swimming swamp bacteria spontaneously organize into crystalsfrom PhysorgMon, 6 Apr 2015, 21:00:44 UTC
- Under the microscope, strong-swimming swamp bacteria spontaneously organize into crystalsfrom The Rockefeller UniversityMon, 6 Apr 2015, 20:00:37 UTC