Location, location, location: Membrane 'residence' gives proteases novel abilities
Related images
(click to enlarge)
Johns Hopkins scientists have discovered a new mode of action for enzymes immersed in cellular membranes. Their experiments suggest that instead of recognizing and clipping proteins based on sequences of amino acids, these proteases' location within membranes gives them the unique ability to recognize and cut proteins with unstable structures. In a report published online Nov. 13 in the new journal eLife, the Johns Hopkins scientists say their study results are the first to shed light on how these enzymes make use of their native environment to function. The particular "cellular scissors" that they studied, known as rhomboid proteases, are unusual among proteases because they cut their target proteins from inside cellular membranes. And because these and other membrane proteases have roles to play in everything from malaria to Parkinson's disease, uncovering their "inside" work could have profound implications for human health, the scientists note.
"The evolution of these proteases, which are found in all types of living organisms, gave cells a whole new set of tools for regulating biology," says principal investigator Sinisa Urban, Ph.D., associate professor of molecular biology and genetics at the Institute for Basic Biomedical Sciences at Johns Hopkins.
Proteases cut proteins for many reasons. The stomach relies on them to indiscriminately break down and digest various proteins people eat. Other proteases are more specialized and help regulate the immune system, for example. Each of these specialized proteases recognizes only specific protein "clients" and only cuts its clients at one specific site.
"Until we did this work, it was thought that specialized proteases decided which proteins to cut based on the presence or absence of a specific sequence of amino acids they recognized," says Urban. "But while most proteases work in watery environments, rhomboid proteases work in oily membranes. Their unique environment suggested to us that they may also have unique properties within the cell."
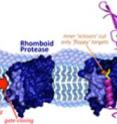
Urban notes that rhomboid proteases are like barrels with a gate that only allows certain proteins inside. Once proteins get past the gate, they interact with the "scissors" inside the barrel and get clipped and released.
For their research, Urban and his team analyzed the activity of rhomboid proteases in microscopic gel-like droplets, which are traditionally used as substitutes for cell membranes, but which are incomplete imitations. To more thoroughly assess the role of the protease's environment in its function, they also developed ways to reassemble rhomboid proteases and their clients in real cell membranes. This allowed them to use cutting-edge biophysical techniques to compare how the enzymes and clients behaved in real membranes versus membrane substitutes.
They report that rhomboid proteases allow more proteins through their gates -- and cut them at different places -- when they are in the gel than when they are in the membrane. "That told us that these proteases are less accurate in recognizing which proteins to cut in the artificial environment than in their natural one," says Urban. "The membrane clearly helps to keep the gate from swinging open and letting unnatural sites to be cut."
The researchers then took a series of different proteins and changed their makeups in a variety of ways to see which ones the rhomboid proteases could cut in living cells. By analyzing dozens of individual changes to various proteins, they found that specific sequences were not the main thing that determined which proteins were cut. Instead, the key factor was whether the protein target was unstable in a watery environment.
Urban explains that when a protein contains a segment that crosses the viscous, oily cell membrane, that segment takes on a curly cue shape, like a slinky, even if it's floppy and shapeless outside the membrane in a watery environment. "Rhomboid proteases have watery inner chambers. If the slinky shape falls apart inside, the protein gets cut. If the slinky shape remains intact, it doesn't get cut."
This insight, says Urban, opens possibilities for better understanding several diseases and ultimately for developing treatments. For example, he says, the protein that builds up in the brain of Alzheimer's patients is a target for another type of membrane-resident protease that isn't well understood either.
Co-authors of the report are Syed Moin and Sinisa Urban from the Johns Hopkins University School of Medicine and the Howard Hughes Medical Institute.
The research was supported by grants from the National Institute of Allergy and Infectious Diseases (AI066025) and the Howard Hughes Medical Institute.
Source: Johns Hopkins Medicine
Other sources
- Location, Location, Location: Membrane 'Residence' Gives Proteases Novel Abilitiesfrom Newswise - ScinewsFri, 16 Nov 2012, 15:01:40 UTC
- Location, location, location: Membrane 'residence' gives proteases novel abilitiesfrom PhysorgFri, 16 Nov 2012, 14:30:46 UTC
- Location, location, location: Membrane 'residence' gives proteases novel abilitiesfrom Science DailyFri, 16 Nov 2012, 14:30:38 UTC