Cell molecule identified as central player in the formation of new blood vessels
Scientists at the University of North Carolina at Chapel Hill School of Medicine have identified a cellular protein that plays a central role in the formation of new blood vessels. The molecule is the protein Shc (pronounced SHIK), and new blood vessel formation, or angiogenesis, is seriously impaired without it. The study, which appeared online Nov. 16, 2011 in the journal Blood, was led by associate professor of cell and molecular physiology at UNC, Ellie Tzima, PhD, who is also a member of the university's Lineberger Comprehensive Cancer Center and the McAllister Heart Institute.
"Angiogenesis is the formation of new blood vessels from existing blood vessels and it's a process that's important during embryonic development and in the development of diseases such as cancer," Tzima said. "So understanding the molecular mechanisms of how blood vessels form is important from the basic science perspective and for understanding and treating disease."
Vascular networks form and expand by sprouting, similar to the way trees grow new branches. The process allows fresh oxygen and nutrients to be delivered to tissues, whether in a developing embryo or a cancerous tumor. Blood vessel formation is spurred by a variety of chemical signals that zoom along complex pathways. Some are cues that come from growth factors, others from the tissue matrix that the cells sit on. This extracellular matrix (ECM) serves the cell in a number of ways, such as supporting the cell's structure, helping to regulate cell-to-cell communication.
The protein Shc, is known to regulate a number of important molecular signaling pathways, but its role in angiogenesis has remained unknown until now, Tzima says. She also points out that Shc is evolutionarily conserved, which indicates its essential importance across species.
"We hypothesized that Shc would be the central player that accepts signals from all of the stimuli that have been previously shown to be important for regulating blood vessel formation and would process them and regulate the cell's response," Tzima said. "And that is what we found -- that Shc coordinates signals, those coming from growth factors as well as from the extracellular matrix."
Tzima suggests that we imagine the cell as a complex highway network with electronic toll plazas through which cars with a transponder can whiz at highway speeds without slowing down. The system works because the transponder's personalized signal is relayed to a computer system that calculates the toll and charges the car's account in a flash. "Shc is the toll plaza, the checkpoint through which signals crucial to blood vessel formation must pass and get coordinated for proper angiogenesis to occur."
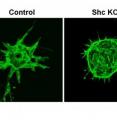
In the study, Tzima and her team found that Shc is required for angiogenesis in zebrafish, mouse and human endothelial cell culture models of blood vessel formation. "The animal studies gave us the broad perspective that Shc may be important to this process," said graduate student and study first-author Daniel T. Sweet. "Zebrafish and mice have previously been used to explore blood vessel formation in vivo. We found that without Shc, blood vessel formation is impaired."
"Then for a closer look we used a cell culture model to determine which endothelial cell processes require Shc for angiogenesis. We found it mediates signals from growth factor receptors and extracellular matrix receptors," Sweet said. "Shc is important for the crosstalk between these processes, meaning that they need to "talk" to each other in order to properly form a tube or to sprout and migrate. That's the exciting thing about this paper."
Tzima notes that elegant genetic models of mice have been used to understand important cellular processes, including angiogenesis. "But if you want to think about designing therapeutics it becomes much more important to understand the molecular mechanism. And this was the strength of the study. We went all the way down to molecular interactions that allowed us to figure out this new angiogenesis pathway."
UNC co-authors with Tzima and Sweet are Zhongming Chen, David M. Wiley, and Victoria L. Bautch. The research was supported by grants from the National Heart, Lung and Blood Institute, American Heart Association and Ellison Medical Foundation.