Scientists crack sparse genome of microbe linked to autoimmunity
Related images
(click to enlarge)
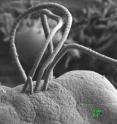
Scientists have deciphered the genome of a bacterium implicated as a key player in regulating the immune system of mice. The genomic analysis provides the first glimpse of its unusually sparse genetic blueprint and offers hints about how it may activate a powerful immune response that protects mice from infection but also spurs harmful inflammation. The researchers, led by Dan Littman, the Helen L. and Martin S. Kimmel Professor of Molecular Immunology at NYU School of Medicine and a Howard Hughes Medical Institute Investigator, and Ivaylo Ivanov, PhD, of Columbia University Medical Center, published their findings in the September 15, 2011, issue of Cell Host and Microbe. The study suggests that the gut-dwelling microorganism, named segmented filamentous bacteria (SFB), is genetically distinct from all 1,200 bacterial genomes studied so far, reflecting its relatively unique role in the gut.
Although SFB was first identified more than 40 years ago, it wasn't until 2009 that Dr. Littman and an international team of collaborators discovered that it can recruit specialized T cells, called Th17 cells, in the small intestine of mice. These potent immune cells, they subsequently found, protected the mice from disease-causing Citrobacter rodentium bacteria, but also made them more susceptible to inflammation and autoimmune arthritis. Those initial results suggested other intestinal bacteria might also regulate immune function.
"What has become clear in the last couple of years is that individual bacteria can specifically influence particular branches of the immune system," says Dr. Littman. In the new study, his team deciphered SFB's 1.57 million letters of DNA, almost 2,000 times smaller than our own genome and about one-third the size of its closest relative.
The microbe's sparse genome lacks many genes needed for its own survival, such as ones for making amino acids and other essential nutrients. As a result, it is dependent on other gut-dwelling bacteria or its host for food, according to the study. The examination of its 1,500 genes, however, suggests it is well adapted to the small intestine, where it clings to the thin lining and may help prevent other microbes from breaching the barrier.
Although the study didn't uncover any definitive signs of the SFB living within us, Dr. Littman suspects the resourceful bacteria have adapted to certain human populations. Even if it isn't found in our intestinal tract, scientists could apply what they have learned to obtain insights into the function of similarly acting microorganisms within us.
"Maybe in humans, there is another bacterium that is different from SFB but behaves functionally in the same way," says Dr. Ivanov, who conducted the latest analysis as a postdoctoral researcher in Dr. Littman's lab.
Recently, Japanese researchers found intestinal bacteria in humans that can boost development of regulatory immune cells in mice, thereby keeping the inflammatory activity of Th17 cells in check. Dr. Littman and his NYU collaborators may have also uncovered a microbe in the intestinal tract of rheumatoid arthritis patients that alters immune function. These emerging results underscore the need to understand how the microbes living in our bodies may impact our health.
"This research brings us the potential genetic mechanisms that trigger differentiation of Th17 cells which we have long believed to have a strong role in the development of autoimmune diseases, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and Crohn's disease," said Steven Abramson, MD, professor, Departments of Medicine and Pathology and director of the Rheumatology Division at NYU Langone Medical Center. "With more than 50 million Americans suffering from at least one autoimmune disease, this research gives scientists and clinicians a greater ability to apply knowledge gained in the laboratory to actual clinical cases, moving it from 'bench-to-beside' to give patients a tremendous advantage and physicians the ability to fine-tune medications and protocols based on patient response."
Recommend this story on Facebook, Twitter,
and Google +1:
Source: NYU Langone Medical Center / New York University School of Medicine
Other sources
- Learning how gut bacteria influence health: Scientists crack sparse genome of microbe linked to autoimmunityfrom Science DailyThu, 15 Sep 2011, 3:30:22 UTC
- Scientists crack sparse genome of microbe linked to autoimmunityfrom PhysorgWed, 14 Sep 2011, 17:30:58 UTC
- Scientists Crack Sparse Genome of Microbe Linked to Autoimmunityfrom Newswise - ScinewsWed, 14 Sep 2011, 15:30:43 UTC