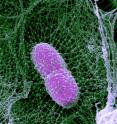
Immune cells deploy traps to catch and kill pathogens
Related images
(click to enlarge)
A new study reveals that two enzymes help immune cells deploy pathogen-killing traps by unraveling and using the chromatin (DNA and its associated proteins) contained in the cells' nuclei to form defensive webs. The study appears online on October 25 in The Journal of Cell Biology (www.jcb.org). Neutrophils, the most common type of white blood cells, are difficult to study because they live for only about six hours. So Arturo Zychlinsky and colleagues, from the Max Planck Institute for Infection Biology in Berlin, created a cell-free system that includes neutrophil nuclei and dollops of cytoplasm from the cells. They found that two enzymes stashed in cytoplasmic granules enter the nucleus and join forces to unwind the chromatin and form neutrophil extracellular traps (NETs), webs of chromatin that catch and kill pathogens. The first to make the move is neutrophil elastase (NE), which promotes chromosome decondensation by breaking down two "histone" proteins that help keep chromatin tightly packaged in the nucleus. Later in the process, myeloperoxidase (MPO) arrives at the nucleus to help NE unravel the chromatin. Exactly how MPO performs its task remains unclear, as its catalytic activity isn't required to decondense chromatin.
The researchers confirmed NE's importance for NET formation by exposing mice to Klebsiella pneumoniae bacteria. Neutrophils hustled to the lungs in control mice and in animals lacking NE. But neutrophils from the mice missing NE couldn't produce NETs to snare the bugs.
An important question to answer now, the researchers say, is how NE and MPO travel to the nucleus. The granules could merge with the nuclear membrane directly or burst and free the enzymes into the cytoplasm, from where they subsequently move to the nucleus.
Source: Rockefeller University Press
Other sources
- Immune cells deploy traps to catch and kill pathogensfrom Biology News NetTue, 26 Oct 2010, 5:01:33 UTC
- Immune cells deploy traps to catch and kill pathogensfrom Science DailyMon, 25 Oct 2010, 18:40:22 UTC
- Immune cells deploy traps to catch and kill pathogensfrom PhysorgMon, 25 Oct 2010, 17:21:20 UTC