New approach doubles 3-D resolution of fluorescence microscopy
Related images
(click to enlarge)
Researchers have developed a new fluorescence microscopy approach that significantly improves image resolution by acquiring three views of a sample at the same time. Their new method is particularly useful for watching the dynamics of biological processes, which can provide insights into how healthy cells work and what goes wrong when diseases occur. In The Optical Society's journal for high impact research, Optica, the researchers apply their multi-view technique in two microscopy modes and use it to image several types of biological samples. For both modes, the researchers demonstrated a volumetric resolution of up to 235 by 235 by 340 nanometers, double the volumetric resolution of traditional methods.
Biologists commonly use fluorescence microscopy to study everything from embryo development to the intricate processes within living cells. However, most fluorescence microscopy methods fail to capture much of the fluorescence emitted from the sample, which not only represents lost information but also reduces image resolution.
"In our work, we captured this previously neglected fluorescence and fused it with the traditional views used in conventional microscopy," said Yicong Wu, staff scientist at the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Maryland, USA, and first author of the Optica paper. "This increases resolution without compromising either temporal resolution or adding additional light to the sample."
Adding a third objective lens
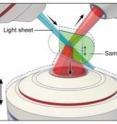
The new multi-view approach helps improve a technique the researchers previously developed called dual-view plane illumination microscopy (diSPIM). Scientists around the world employ commercial versions of diSPIM, which uses a thin sheet of light and two objectives lenses to excite and detect fluorescence.
"The main motivation of this new research was that the resolution in diSPIM was limited by the numerical aperture of the upper lenses, and fluorescence emitted in the direction of the coverslip is not captured," explained Hari Shroff, leader of the research team. "We reasoned that if we could simultaneously image this neglected signal by adding a higher numerical aperture lens that acquired the bottom view, then we could boost the lateral resolution."
In the improved diSPIM microscopy technique, each light sheet is tilted at a 45-degree angle relative to an additional lower objective lens. In its current design, the researchers swept the lower objective's plane of focus through the sample to image the previously unused fluorescence, but this mechanical scanning could be replaced with a passive optic in future versions of the microscope. Using the multi-view approach improved the lateral, or horizontal, resolution of diSPIM to about 235 nm.
The researchers also implemented the new technique in wide-field mode by scanning the three objectives through a sample simultaneously to produce three individual 3D views. With this mode, the multi-view method improved axial, or Z-axis, resolution, to about 340 nm, an increase of 45%.
Merging three views into one
Whether acquired in wide-field or light-sheet mode, the three views must be precisely aligned and also cleaned up with an image processing technique known as deconvolution.
"One helpful trick was to deconvolve each view first to increase image quality, contrast, and so forth, which then allowed accurate registration of the three views," said Wu. "In wide-field mode, we further aided registration of the images by adding fluorescent beads to the samples as point of reference." He added that collaboration with Patrick La Riviere's research group at the University of Chicago was essential in thinking through and testing this deconvolution method.
The researchers demonstrated the multi-view technique by imaging biological samples and were able to see detailed features not typically observable. For example, the wide-field multi-view microscope clearly resolved the spherical protein shell present when Bacillus subtilis forms a spore and also allowed the researchers to observe the dynamics of organelles inside cells. In light-sheet mode, they clearly saw the 3D dynamic nature of tiny protrusions on living white blood cells when they acquired 150 triple-view images over 40 minutes.
Although other methods have been used to capture multiple views sequentially, this new method improves spatial resolution without introducing additional illumination or compromising temporal resolution relative to conventional imaging. This is important because additional light can be damaging, even deadly to living cells, and the temporal resolution is needed to capture fast processes.
The research team is now exploring additional biological applications for the new system and is working to extend the method to other microscope modalities, such as confocal microscopy.
Source: The Optical Society
Articles on the same topic
- New microscopy system captures 'lost' fluorescence, improving resolutionSat, 13 Aug 2016, 14:11:32 UTC
Other sources
- New microscopy system captures 'lost' fluorescence, improving resolutionfrom Science DailySat, 13 Aug 2016, 14:02:16 UTC
- New approach doubles 3-D resolution of fluorescence microscopyfrom Science DailyFri, 12 Aug 2016, 14:01:27 UTC
- New approach doubles 3-D resolution of fluorescence microscopyfrom PhysorgThu, 11 Aug 2016, 14:21:15 UTC