Graphene nanocomposite a bridge to better batteries
Researchers with the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have created a graphene and tin nanoscale composite material for high-capacity energy storage in renewable lithium ion batteries. By encapsulating tin between sheets of graphene, the researchers constructed a new, lightweight "sandwich" structure that should bolster battery performance. "For an electric vehicle, you need a lightweight battery that can be charged quickly and holds its charge capacity after repeated cycling," says Yuegang Zhang, a staff scientist with Berkeley Lab's Molecular Foundry, in the Inorganic Nanostructures Facility, who led this research. "Here, we've shown the rational design of a nanoscale architecture, which doesn't need an additive or binder to operate, to improve battery performance."
Graphene is a single-atom-thick, "chicken-wire" lattice of carbon atoms with stellar electronic and mechanical properties, far beyond silicon and other traditional semiconductor materials. Previous work on graphene by Zhang and his colleagues has emphasized electronic device applications.
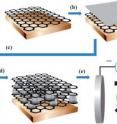
In this study, the team assembled alternating layers of graphene and tin to create a nanoscale composite. To create the composite material, a thin film of tin is deposited onto graphene. Next, another sheet of graphene is transferred on top of the tin film. This process is repeated to create a composite material, which is then heated to 300˚ Celsius (572˚ Fahrenheit) in a hydrogen and argon environment. During this heat treatment, the tin film transforms into a series of pillars, increasing the height of the tin layer.
"The formation of these tin nanopillars from a thin film is very particular to this system, and we find the distance between the top and bottom graphene layers also changes to accommodate the height change of the tin layer," says Liwen Ji, a post-doctoral researcher at the Foundry. Ji is the lead author and Zhang the corresponding author of a paper reporting the research in the journal Energy and Environmental Science.
The change in height between the graphene layers in these new nanocomposites helps during electrochemical cycling of the battery, as the volume change of tin improves the electrode's performance. In addition, this accommodating behavior means the battery can be charged quickly and repeatedly without degrading — crucial for rechargeable batteries in electric vehicles.
"We have a large battery program here at Berkeley Lab, where we are capable of making highly cyclable cells. Through our interactions in the Carbon Cycle 2.0 program, the Materials Science Division researchers benefit from quality battery facilities and personnel, along with our insights in what it takes to make a better electrode," says co-author Battaglia, program manager in the Advanced Energy Technology department of Berkeley Lab's Environmental and Energy Technologies Division. "In return, we have an outlet for getting these requirements out to scientists developing the next generation of materials."
Source: DOE/Lawrence Berkeley National Laboratory
Articles on the same topic
- Rice scientists build battery in a nanowire Fri, 29 Jul 2011, 17:05:34 UTC
Other sources
- Scientists build battery in a nanowirefrom PhysorgFri, 29 Jul 2011, 17:01:46 UTC
- Graphene nanocomposite a bridge to better batteriesfrom Science DailyThu, 28 Jul 2011, 2:30:22 UTC
- Graphene nanocomposite a bridge to better batteriesfrom PhysorgWed, 27 Jul 2011, 20:32:48 UTC