Team solves decades-old molecular mystery linked to blood clotting
Blood clotting is a complicated business, particularly for those trying to understand how the body responds to injury. In a new study, researchers report that they are the first to describe in atomic detail a chemical interaction that is vital to blood clotting. This interaction – between a clotting factor and a cell membrane – has baffled scientists for decades. The study appears online in the Journal of Biological Chemistry.
"For decades, people have known that blood-clotting proteins have to bind to a cell membrane in order for the clotting reaction to happen," said University of Illinois biochemistry professor James Morrissey, who led the study with chemistry professor Chad Rienstra and biochemistry, biophysics and pharmacology professor Emad Tajkhorshid. "If you take clotting factors off the membrane, they're thousands of times less active."
The researchers combined laboratory detective work with supercomputer simulations and solid-state nuclear magnetic resonance (SSNMR) to get at the problem from every angle. They also made use of tiny rafts of lipid membranes called nanodiscs, using an approach developed at Illinois by biochemistry professor Stephen Sligar.
Previous studies had shown that each clotting factor contains a region, called the GLA domain, which interacts with specific lipids in cell membranes to start the cascade of chemical reactions that drive blood clotting.
One study, published in 2003 in the journal Nature Structural Biology, indicated that the GLA domain binds to a special phospholipid, phosphatidylserine (PS), which is embedded in the membrane. Other studies had shown that PS binds weakly to the clotting factor on its own, but in the presence of another phospholipid, phosphatidylethanolamine (PE), the interaction is much stronger.
Both PS and PE are abundant in the inner – but not the outer – leaflets of the double-layered membranes of cells. This keeps these lipids from coming into contact with clotting factors in the blood. But any injury that ruptures the cells brings PS and PE together with the clotting factors, initiating a chain of events that leads to blood clotting.
Researchers have developed many hypotheses to explain why clotting factors bind most readily to PS when PE is present. But none of these could fully explain the data.
In the new study, Morrissey's lab engineered nanodiscs with high concentrations of PS and PE, and conducted functional tests to determine if they responded like normal membranes.
"We found that the nanodisc actually is very representative of what really happens in the cell in terms of the reaction of the lipids and the role that they play," Morrissey said.
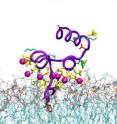
Then Tajkhorshid's lab used advanced modeling and simulation methods to position every atom in the system and simulated the molecular interactions on a supercomputer. The simulations indicated that one PS molecule was linking directly to the GLA domain of the clotting factor via an amino acid (serine) on its head-group (the non-oily region of a phospholipid that orients toward the membrane surface).
More surprisingly, the simulations indicated that six other phospholipids also were drawing close to the GLA domain. These lipids, however, were bending their head-groups out of the way so that their phosphates, which are negatively charged, could interact with positively charged calcium ions associated with the GLA domain. (Watch a movie of the simulation.)
"The simulations were a breakthrough for us," Morrissey said. "They provided a detailed view of how things might come together during membrane binding of coagulation factors. But these predictions had to be tested experimentally."
Rienstra's lab then analyzed the samples using SSNMR, a technique that allows researchers to precisely measure the distances and angles between individual atoms in large molecules or groups of interacting molecules. His group found that one of every six or seven PS molecules was binding directly to the clotting factor, providing strong experimental support for the model derived from the simulations.
"That turned out to be a key insight that we contributed to this study," Rienstra said.
The team reasoned that if the PE head-groups were simply bending out of the way, then any phospholipid with a sufficiently small head-group should work as well as PE in the presence of PS. This also explained why only one PS molecule was actually binding to a GLA domain. The other phospholipids nearby were also interacting with the clotting factor, but more weakly.
The finding explained another mystery that had long daunted researchers. A different type of membrane lipid, phosphatidylcholine (PC), which has a very large head-group and is most abundant on the outer surface of cells, was known to block any association between the membrane and the clotting factor, even in the presence of PS.
Follow-up experiments showed that any phospholipid but PC enhanced the binding of PS to the GLA domain. This led to the "ABC" hypothesis: when PS is present, the GLA domain will interact with "Anything But Choline."
"This is the first real insight at an atomic level of how most of the blood-clotting proteins interact with membranes, an interaction that's known to be essential to blood clotting," Morrissey said. The findings offer new targets for the development of drugs to regulate blood clotting, he said.
Source: University of Illinois at Urbana-Champaign
Other sources
- Decades-old molecular mystery linked to blood clotting solvedfrom Science DailyTue, 31 May 2011, 23:30:54 UTC
- Researchers solve decades-old molecular mystery linked to blood clottingfrom PhysorgTue, 31 May 2011, 16:31:21 UTC