Penn study finds way to prevent protein clumping characteristic of Parkinson's disease
Related images
(click to enlarge)
Researchers at the University of Pennsylvania School of Medicine have identified a protein from a most unlikely source -- baker's yeast -- that might protect against Parkinson's disease. More than a million Americans suffer from Parkinson's disease, and no treatments are available that fundamentally alter the course of the condition. By introducing the yeast protein Hsp104 into animal models of Parkinson's disease, researchers prevented protein clumping that leads to nerve cell death characteristic of the disorder. "Yeast express a protein called Hsp104, which is able to reverse protein aggregation," says James Shorter, PhD, Assistant Professor of Biochemistry and Biophysics. "However, for reasons that are unclear, Hsp104 is not found in mammals. We wondered if introducing Hsp104 into mammals could help with diseases connected with protein aggregation."
These findings will be published in the September 2008 issue of The Journal of Clinical Investigation and appeared online August 14, 2008
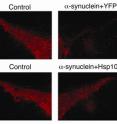
Clinicians do not fully understand the process and cause of Parkinson's disease. However, researchers believe that a protein called alpha-synuclein misfolds and clumps in many forms of the disease, and that this process is intimately tied to the selective death of dopamine-producing neurons that results in Parkinson's disease.
In this study, researchers found that Hsp104 could partially reverse alpha-synuclein aggregation in test-tube experiments. Remarkably, rats expressing Hsp104 showed lower levels of alpha-synuclein aggregation and alpha-synuclein-induced toxicity of neurons. This result is significant because the rat model used recreates the selective loss of dopamine-producing nerve cells in the region of the brain affected in Parkinson's disease, say the investigators.
"This study represents an important preliminary step," says Shorter. "One thing we'd like to do next is to treat an animal model which already has considerable quantities of alpha-synuclein aggregates to see if Hsp104 can actually reverse the process in the rat brain."
Co-authors in addition to Shorter are Christophe Lo Bianco of the Wallenberg Neurosciences Center in Lund, Sweden and the Brain Mind Institute in Lausanne, Switzerland; Etienne Regulier, Hilal Lasheul, and Patrick Aebischer, also of the Brain Mind Institute; Takeshi Iwatsubo at the University of Tokyo; and Susan Lindquist of the Whitehead Institute for Biomedical Research, Cambridge, MA. The Michael J. Fox Foundation, European Molecular Biology Organization, Swedish Parkinson's Foundation, Swiss National Science Foundation, American Heart Association, University of Pennsylvania Institute on Aging, and the National Institute of Health Director's New Innovator Award provided funding for this research.
Source: University of Pennsylvania School of Medicine
Articles on the same topic
- Lessons from yeast: A possible cure for Parkinson's disease?Thu, 14 Aug 2008, 21:56:26 UTC
Other sources
- Study finds way to prevent protein clumping characteristic of Parkinson's diseasefrom PhysorgFri, 15 Aug 2008, 17:28:15 UTC
- Lessons From Yeast: A Possible Cure For Parkinson's Disease?from Science DailyThu, 14 Aug 2008, 22:21:07 UTC
- Lessons from yeast: A possible cure for Parkinson's disease?from PhysorgThu, 14 Aug 2008, 21:56:06 UTC