Tethered molecules act as light-driven reversible nanoswitches
Our ability to see is based on molecules in the eye that flip from one conformation to another when exposed to visible light. Now, a new technique for attaching light-sensitive organic molecules to metal surfaces allows the molecules to be switched between two different configurations in response to exposure to different wavelengths of light. Because the configuration changes are reversible and can be controlled without direct contact, this technique could enable applications that can be controlled at the molecular scale. The technology has been suggested as a possible basis for molecular motors, artificial muscles, and molecular electronics. The research results, obtained by a team led by Paul S. Weiss, distinguished professor of chemistry and physics at Penn State University and James M. Tour, Chao professor of chemistry at Rice University, are reported in the June 2008 issue of the journal Nano Letters.
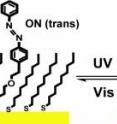
Until now, progress was impeded because, when such molecules were attached to surfaces, they no longer could be switched back and forth, as they could be when they were in solution. The new technique uses a change in the shape of an azobenzene molecule in response to light to provide two different states. The azobenzene molecule consists of a bridge of two nitrogen atoms attached to one another by a double bond, with each nitrogen atom also bound to a benzene ring. The two benzene rings can be on the same side of the molecule (cis configuration) or on opposite sides (trans configuration). When the molecule absorbs energy, in the form of light, it can change between cis and trans configurations in a process called photoisomerization. "This mechanism is essentially the same that we use in our eyes for vision," said Weiss. "The molecule responds to light by making a change that can be harnessed. In the eye, the change causes a neural impulse."
The photoisomerization of azobenzene is understood well in solution, but the molecule must be attached to a surface in order to provide a useful molecular switch or component of a motor. Previous attempts to accomplish the switching with attached molecules were unsuccessful, either due to interactions between the molecule and the surface to which it was attached or to interferences between adjacent molecules. "To overcome the difficulty of reversible photoisomerization of molecules on surfaces, we used a carefully designed 'tether' to isolate the functional molecules from one another and from the metal surface," said Weiss. "We isolated the tethered molecules in the surrounding matrix on a self-assembled monolayer and confirmed this isolation using molecular-resolution scanning tunneling microscopy."
When the tethered molecules were exposed to ultraviolet light in a specially built scanning tunneling microscope, they switched from the trans to the more-compact cis state. This switch was confirmed by an apparent decrease in height of the molecule above the surrounding surface. The researchers further found that exposure to visible light caused a transition back to the more-extended trans state.
Weiss points out that this research advance is just the first step in designing a device that can be driven or actuated by such molecular change. In order to perform useful work as a switch or nanoscale-drive motor, it will be necessary to coordinate the motion of multiple molecules and to build moving parts into some sort of assembly. According to Weiss, further research by the team already has found some surprises when the molecules are lined up to work in unison, like a chorus line.
Source: Penn State
Other sources
- Tethered Molecules Act As Light-driven Reversible Nanoswitchesfrom Science DailyWed, 25 Jun 2008, 2:28:13 UTC
- Tethered molecules act as light-driven reversible nanoswitchesfrom PhysorgMon, 23 Jun 2008, 23:20:30 UTC