Inhibiting cholesterol-associated protein reduces high-risk blockages in arteries
Related images
(click to enlarge)
Using the drug darapladib, researchers at the University of Pennsylvania School of Medicine and colleagues have inhibited a cholesterol-and immune system-associated protein, thereby reducing the development of heart-disease plaques that may cause death, heart attacks, and strokes in a pig model of atherosclerosis and diabetes. The study appeared online this week in Nature Medicine. "We've used a model that closely mimics clinical disease," says first author Robert L. Wilensky, MD, Director of Experimental Interventional Cardiology and Professor of Medicine at the Penn Cardiovascular Institute. "The study shows that darapladib is useful in reducing atherosclerosis but more importantly those blockages that are thought to cause death and heart attacks."
Atherosclerosis, or hardening of the arteries, is the most common cause of heart attack, stroke, and death from cardiovascular disease, and has long been thought of as a type of chronic inflammation. An early first step in the build-up of the plaques associated with atherosclerosis is the accumulation of low-density lipoproteins (LDLs), the "bad" cholesterol, on artery walls. When LDLs are oxidized by the body, they attract immune cells and lipids to the site of the build-up.
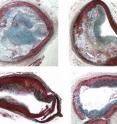
Problems arise when the plaques grow to form a lesion characterized by a thin fibrous cap and a lipid-filled core of dying cells. These unstable plaques are prone to rupture, which can then lead to heart attack, stroke, and death.
A molecule called lipoprotein-associated phospholipase A2 (Lp-PLA2) is connected with LDLs circulating in the blood. Elevated levels of Lp-PLA2 in the blood are associated with an increased risk of heart disease events and are related to the development of the necrotic core of plaques. Darapladib specifically inhibits Lp-PLA2.
"The results are exciting," says Wilensky. "First, darapladib reduced the overall amount and size of plaques that block the coronary arteries of animals in the study. More importantly, it reduced the number and size of the type of advanced plaques that cause heart attacks and strokes. "
These advanced plaques have a thin cap and large core filled with cellular debris from inflammatory-immune cells that engorge themselves on cholesterol. If unstable plaques come into contact with blood, blood clots that develop from this contact constrict flow, which can lead to stroke and heart attack. Darapladib stabilizes these dangerous plaques by decreasing the size of the core and reducing the number of inflammatory-immune cells present within the plaque. Darapladib also decreased the expression of genes involved in enlisting immune cells involved in the inflammatory response associated with atherosclerosis.
"The aha moment came when we saw the profound difference in plaque composition in animals given medication versus those not given darapladib, although the high cholesterol levels in the pig model remained the same in both groups," says Wilensky. "This study took cholesterol out of the equation and let us evaluate the effects of inflammation on the development of atherosclerosis."
Recently, darapladib has been tested in a human clinical trial in Europe, which showed similar findings. GlaxoSmithKline (GSK) Inc., who provided the darapladib for the study, is planning a Phase 1 safety and efficacy trial with darapladib in humans in the near future. Penn will be one site in this proposed multi-center clinical trial. This study was supported, in part, by funding from GlaxoSmithKline, over the last two years totaling about $1.5 million, through an industry-academic alliance called the Alternative Drug Discovery Initiative at the Penn School of Medicine. Co-author Emile Mohler, III, has a position on a steering committee as a National Coordinator for the Phase III GSK trial for darapladib.
Source: University of Pennsylvania School of Medicine
Other sources
- Study may lead to new heart disease drugsfrom UPIWed, 24 Sep 2008, 18:07:08 UTC
- Inhibiting cholesterol-associated protein reduces high-risk blockages in arteriesfrom PhysorgMon, 22 Sep 2008, 20:07:10 UTC